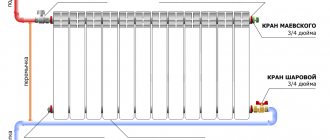
Did you like the article? Stay tuned for new ideas and useful auto tips in our channel. Subscribe to us on Yandex.Dzen. Subscribe.
The radiator is a technically complex unit, on which the efficiency and uninterrupted operation of the engine depend. Considering this, it is not recommended to carry out diagnostics and repair work on your own.

Why is it necessary to rinse and how often
In apartment buildings connected to centralized heat supply networks, heating systems are flushed annually and strictly according to a schedule that meets the requirements of SNiP. In the private sector, this procedure is performed as needed.
It will be much cheaper to flush the system annually in a private house, performed in the inter-heating period, than to allow dirt and sediments to accumulate in it for several years, waiting for the overlap of most of the pipeline cross-section.
In urban boiler houses, water treatment is regularly used to clean the coolant, but the unsatisfactory condition of the networks leads to constant water pollution. It is not easy for city utilities to cope with such a problem, which is why sometimes summer temporary outages of hot water occur.
The owners of individual dwellings fill the heating system with plain water from the water supply system without any preparation, in this case the only precaution is to install a filter at the water inlet to the house. Regular and timely flushing of the heating system in a private house allows you to increase the service life and increase the efficiency of the boiler, pipes and radiators, prevents the formation and attachment of salts and scale to their walls, leading to destruction
The filter installed in the boiler piping circuit is able to protect the heating equipment only from small impurities that are initially in the water and do not cause any special problems.
If the heating systems are not flushed for a long time, then the resulting deposits are even more dangerous and lead to a significant decrease in the efficiency of the heating network, reducing the inner diameter of the pipes and, accordingly, the throughput. In this regard, the hydraulic resistance of the pipeline increases, and the batteries do not receive enough heat required for normal space heating. Scale in the radiators and heat exchanger significantly reduces their heat transfer performance. The heat generator has to consume more fuel to increase the energy of the heat carrier and, as a result, increase the temperature in the living room.


Cleaning the heating system is usually the last-in-line procedure to be followed by a preoccupied homeowner. Often not understanding what the matter is, the owner raises the temperature of the coolant by simply turning the handle of the boiler, causing an increase in fuel consumption.
Protective oxide film - for how long?
Very often in advertising brochures and on the websites of aluminum radiator manufacturers (especially our Russian factories), you can find the following statement: “During the production of our aluminum radiators, a strong protective film of aluminum oxide is formed on their inner surface, which reliably protects the radiator from the internal corrosion ".
Firstly, the manufacturers of Russian aluminum radiators, 100% of which are made by extrusion (and not because it is better, but because the organization of such production requires incommensurably less costs than the organization of the foundry production of aluminum radiators - for more details on comparing the method of extrusion and casting of aluminum radiators, see the article "Construction of aluminum radiators"
) present the formation of this protective film as one of the advantages of the extrusion method they use for the production of aluminum radiators.
In fact, this oxide film forms on absolutely any aluminum surface - no matter what method (casting or pressing) the aluminum section was made by.
Looking into any school chemistry textbook, we will find information that upon contact with air, aluminum forms a thin non-porous oxide film (chemical formula Al2O3), which protects this metal from further oxidation, which determines its high anti-corrosion resistance.
And if crystal clear water with a neutral pH and without any mechanical impurities would flow through the central heating pipes, then it would be so - the formed oxide film would protect the aluminum alloy from further oxidation for a long time and would really prevent its destruction.
But it's not a secret for anyone that the quality of water in our Russian heating systems is EXTREMELY LOW, and the water contains just a HUGE AMOUNT of these very polluting particles (sand, small stones, particles of rust and lead scale and a lot of other interesting things). These very mechanical particles, passing through the aluminum radiator at a fairly high speed, cause abrasive wear of the inner surface, and the first thing they do is mechanically destroy this most notorious protective film, and only then they are taken for the aluminum wall itself (aluminum, as you know, is a very soft metal that is very easy to scratch).
In addition, much more active processes of its chemical destruction are added to the processes of mechanical destruction of this very protective oxide film. In the same textbook on chemistry, you can read that aluminum oxide has a high "amphotericity" - that is, the ability to enter into chemical reactions with both alkalis and acids to form water-soluble salts that do not remain on the metal, but enter the coolant.
And since hot water in the central system of heating networks, in addition to a high content of mechanical particles, also has a very unstable acid-base balance, very far from neutral indicators, then these chemical reactions proceed very actively - destroying this very protective oxide film and exposing aluminum.
Surprisingly, but a fact - if sulfuric or nitric acid would flow to the heating pipes instead of water, then this protective film would remain intact, since aluminum oxide does not react with these two so poisonous acids!
But back to our aluminum radiator, not sulfuric, but water heating. :))
In such an aggressive environment, even in order to destroy a radiator wall made of aluminum alloy, it may take only 4-5 years (!) - given the fact that manufacturers try to make aluminum walls as thin as possible (after all, this is one of the main advantages of this type of radiator are the subtlety and grace of the design), and much more active processes of chemical corrosion are added to the processes of rather slow mechanical abrasion.
What can we say about a thin oxide film - not even a trace remains of it after a few months! Therefore, reading the statements of some who are either not too literate, or not too honest is simply ridiculous.
Consequences of clogging


Regardless of what the source of clogging of the heating pipe is, the outcome is almost always the same:
- after a certain moment, the pipes are clogged;
- the movement of water in the pipes is reduced and later even the water pump will not be able to pump water through this system.
Things are much worse with thermosyphon heating, where there is no such pump. As a rule, after clogging, heat is not allowed through and the pipes remain cold. And this is only part of the trouble. In addition, the boiler itself begins to heat up strongly, which can lead to its breakdown.
Some owners carry out an annual cleanup of the blockages of such a system by changing the water. In other words, the old unclean, rusty water is drained and filled with new. And this is reasonable, because when the old water is drained, a small amount of chips and rust leaves it. But there is also an opposite side. Iron and oxygen are needed for rust to appear. If the pipe is metal, then iron is always present in it, but oxygen is contained in the water. As a rule, when you do not change the liquid in the heating system for a long time, the oxygen content in it decreases significantly, which means that the rusting process stops. With a constant change of water, on the contrary, its activation occurs. Summing up a small summary, we can say one thing - this method helps to get rid of a small amount of rust, but, on the other hand, we only speed up the new process of its formation.
Features of the use of inhibitors


Specially developed reagents for heating systems have the following features:
- Protects all types of metals from corrosion;
- Reduce the adhesion of water-soluble components;
- Prevent the formation of precipitation of insoluble substances in the heating system;
- Designed for use at temperatures above 100 ° C;
- Effective protection period - 5 years;
- The regent should occupy 2 - 2.5% of the total volume of the coolant in the heating system. This significantly reduces the cost of protecting heating systems;
- The additives contain volatile substances that, when evaporated from water, create a protective layer on surfaces that does not come into direct contact with the coolant;
- The additives do not contain harmful substances;
- Slows down the development of bacteria and algae.
Elimination of radiator defects
The condition of the radiator should be checked regularly. This is especially important before a long trip. When a leak appears in the radiator due to corrosion, it is necessary to use special sealants or cold welding. Small leaks in the cooling system will help fix the seals. For these purposes, the sealant is poured into the tank of the cooling system. In contact with air, such substances solidify, forming a polymer film that reliably closes the leak. Cold welding is a more difficult type of repair. It is used in the presence of large cracks.


Heat-resistant adhesive sealants that resemble plasticine are applied to the damaged surface. The sealant sets within a few minutes, but full hardening may occur much later. Sometimes this takes a whole day. These remedies are, in fact, emergency. In the near future it will be necessary to contact a car service for more substantial repairs, otherwise the radiator will have to be replaced with a new one. Even if "cold welding" can last for several years, it is still not worth the risk.
How does corrosion in pipes appear and what does it lead to?


As the water temperature rises for every 10 ° C, its ability to cause corrosion doubles and the ability to dissolve CaCO3 and CaSO4 salts decreases, which leads to accelerated scale formation.
However, it is not only reactions between different chemical elements that harm heating systems. Substances that are dissolved in any water have the ability to settle and attach to the walls of streams.
These chemical processes contribute to the formation of rust and scale in the heating system, which reduces pipe clearance and heat transfer.
A corrosion inhibitor is used to prevent or slow down corrosion processes in heating systems. Various additives and reagents are used to reduce the formation of scale.
Rust control


In order for rust not to spoil the heating, you need to prepare the system for start-up in advance. To this end, you need not just pour water into the pipe, but add a special antifreeze to it. Its action is the same as in the engine fluid, that is, it guarantees good heat transfer through the pipes, and also forms the protection of metal surfaces from oxidative processes and prevents the origin of lime deposits and other deposits. This alternative is quite expensive, but makes it possible to forget about constant cleaning.
The entire cleaning stage is relatively simple and does not require complex techniques. The process will proceed as follows:
- pipe cleaning;
- cleaning the heating boiler itself.
Pipe cleaning


The easiest way to clean the heating system is to use chemicals. All we need is to buy a product that can dissolve rust and other types of deposits.
Ordinary citric acid, which every housewife has, can act as such a remedy. It must be dissolved in water, it is advisable to use a three-liter jar, since a large amount gives a greater effect. All this solution must be poured into the heating system. Subsequently, it is immediately necessary to light the boiler, set the temperature to a high mark, and it remains to wait twenty-four hours. Later we drain this water. We wash the pipes by filling and re-draining clean water.
Another similar technique is the use of food vinegar. It takes a lot to achieve the best effect. But there is also a safer option - this is the use of hydrochloric acid, mainly 10 or 20%. This chemical is excellent at cleaning pipes. But you need to be careful with this substance, since too high a concentration can significantly damage the heating system.


This operation is suitable only for small blockages. If the pipes are clogged thoroughly, then the compressor will help out. Most often, this method is called hydropneumatic cleaning.
The process will proceed as follows:
- we connect the compressor to the heating system;
- we connect the compressor to the pipe and start;
- flushing begins with a simultaneous combination with pneumatic blows;
- disconnect the pipe going to the boiler (bottom);
- we put some container next to it so that dirty water flows there;
- clean water must constantly flow into the riser (during the discharge of unclean water).
The compressor is expensive and if you don't want to spend money, then you can dismantle the radiators (each separately). That is, they are flushed under tremendous water pressure.
Boiler cleaning
There may be deposits in the boiler itself. In addition, there are more of them here than in pipes. The fact is that it heats up very much, due to which the process is accelerated.
Chemicals are used here. The whole work is quite simple: you need to disconnect the heating pipes, take a pump that is combined with a boiler and water is admitted through it, with chemistry added in advance. We drain all the dirty water and then rinse it with clean water.
Having mastered all the considered tips, you will be able to flush the heating system with full confidence on your own.
Types of radiators
Radiators may differ in assembly method, material of manufacture and optional components. They can be divided into the following options:
- Prefabricated radiators. In them, the connection of the components was carried out mechanically. Such an assembly is notable for its affordable cost, the joints of such models needed sealing gaskets, which are resistant to antifreeze and temperature extremes;
- Copper radiators. They are more expensive, but damage to them can be easily repaired by sealing;
- Aluminum radiators. Such products are more durable and reliable, but aluminum gives off heat worse than copper.


Selection and recommendations for the use of an inhibitor for the heating system
One or another inhibitor must be selected based on several indicators:
- An open or closed expansion tank is used;
- Type of construction materials used: ferrous metals, alloys based on copper or aluminum;
- PH indicator of water;
- Indicators of "hardness" of water (the amount of dissolved salts in the coolant).
Depending on the hardness and acidity of the coolant, as well as the characteristics of the heating system, it is necessary to choose an inhibitor of a certain composition. The following additive compositions are distinguished:
- Orthophosphate. The reagent forms a protective film, causes the precipitation of salts, in case of their large amounts. It is necessary to add to the coolant based on the proportion of 10 - 20 mg / l. It is used in heating systems where elements are made of ferrous metals with a water Ph level of less than 7.5 units. Chlorine concentration in water of 300 mg / l and more levels the effectiveness of orthophosphate and leads to metal corrosion. Can be used in combination with zinc polyphosphate or phosphanate additive;
- Polyphosphates. They are used to protect pipelines made of ferrous metals with water Ph up to 7.5 units. No water softening is required when using polyphosphate. The amount of chlorine also does not affect the properties of this inhibitor. The effectiveness of the action of polyphosphates is increased with the help of zinc. The optimal amount is 10 - 20 mg / l;
- Phosphonates. It is used only in combination with zinc, orthophosphates or polyphosphates. The composition will be effective at a concentration of 10 - 20 mg / l and at Ph 7 - 9. Protection of ferrous metals is provided by the addition of calcium;
- Molybdate. The reagent protects ferrous and aluminum alloys. It is necessary to add to the coolant at the rate of 75 - 150 mg / l, in order to reduce the amount of the composition without reducing the efficiency, the addition of phosphorus components is required. Recommended water Ph is 5.5 - 8.5. Hard water causes the molybdate to precipitate. Chlorine and sulfur impurities neutralize the use of molybdate, but without the occurrence of pitting corrosion;
- Silicate. It is used for soft water at a concentration of 10 - 20 mg / l. Provides protection for systems made of ferrous metals and copper alloys with water having a Ph 7 and higher. A protective coating forms on surfaces over several weeks;
- Zinc. It is used as an additive to other additives: orthophosphates, polyphosphates, phosphonates, molybdates. And also with combinations of inhibitors that do not contain zinc: orthophosphate / polyphosphate, orthophosphate / molybdate, a mixture of phosphonates in the amount of 0.5 - 2 mg / l. Zinc strengthens the protective film and reduces the amount of the main inhibitor. If the Ph of water exceeds 7.5, it is necessary to use zinc stabilizers;
- Benzotriazole. The required concentration is 1 - 2 mg / l in water with Ph 6 - 9 for the protection of copper alloys;
- Tolitriazole. A benzotriazole analog;
- Calcium orthophosphate. Used to eliminate the adhesion of calcium phosphate deposits. The content of calcium orthophosphate in water should be 10-15 mg / l;
- Polyacrylates, polymaleates, hydrolyzed polyacrylamides and acrylate substances. Used for biological contamination. The optimal concentration is 2-3 mg / l;
- Chlorine and bromine are used to kill microorganisms.A concentration at the level of 0.1 - 0.5 mg / l is sufficient. Chlorine is effective only in water with a Ph below 8. If the pH exceeds this value, bromine is used;
- Zeolites. Used to soften water;
- Nitrite. Used in closed systems, it causes the formation of a stable iron oxide film on the surface. Effective in concentrations of 250-1000 mg / l and increasing Ph up to 9 - 9.5, by adding borax. The amount of nitrite can be reduced to 300 mg / l using the same amount of molybdate. Nitrites lend themselves to decomposition by bacteria, therefore, in the complex, it is also necessary to use a non-oxidizing bactericide, copper corrosion inhibitors and a polymer dispersant;
- Alkalis (caustic soda, ash). Used to increase the Ph of water to 9 - 10.5 units.
Radiator and corrosion
When the cooling system stops functioning, it is necessary to carefully examine it to determine the defect. Spent refrigerant can cause corrosion on the radiator surface. It begins to ionize almost immediately after refueling. In this case, the liquid begins to destroy surfaces of metal, to which it can come into contact, moving through the system.
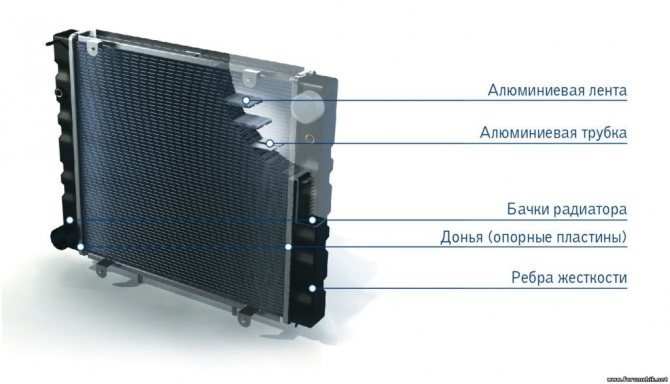

Old ionized refrigerant can cause damage after just a few weeks of operation. When the radiator starts to leak, it can be due to mechanical damage or corrosion. It can occur for many reasons, including poor quality coolant, the presence of salts in the water, or damage to the protective coating of the device. Timely elimination of the defect will help prolong the performance of the automotive part.