Methods for producing hydrogen in industrial conditions
Extraction by methane conversion
... Water in a vapor state, preheated to 1000 degrees Celsius, is mixed with methane under pressure and in the presence of a catalyst. This method is interesting and proven, it should also be noted that it is constantly being improved: the search for new catalysts, cheaper and more effective, is underway.
Consider the oldest method for producing hydrogen - coal gasification
... Provided there is no air access and a temperature of 1300 degrees Celsius, coal and water vapor are heated. Thus, hydrogen is displaced from water, and carbon dioxide is obtained (hydrogen will be at the top, carbon dioxide, also obtained as a result of the reaction, is at the bottom). This will be the separation of the gas mixture, everything is very simple.
Obtaining hydrogen by electrolysis of water
is considered the simplest option. For its implementation, it is necessary to pour a soda solution into the container, and also place two electrical elements there. One will be charged positively (anode) and the other negatively (cathode). When current is applied, hydrogen will go to the cathode and oxygen to the anode.
Obtaining hydrogen by the method partial oxidation
... For this, an alloy of aluminum and gallium is used. It is placed in water, which leads to the formation of hydrogen and alumina during the reaction. Gallium is necessary for the reaction to take place in full (this element will prevent aluminum from oxidizing prematurely).
Recently acquired relevance method of using biotechnology
: under the condition of a lack of oxygen and sulfur, chlamydomonas begin to intensively release hydrogen. A very interesting effect that is now being actively studied.

Do not forget another old, proven method of hydrogen production, which consists in using different alkaline elements
and water. In principle, this technique is feasible in a laboratory setting provided the necessary safety measures are in place. Thus, in the course of the reaction (it proceeds with heating and with catalysts), a metal oxide and hydrogen are formed. It remains only to collect it.
Get hydrogen by interaction of water and carbon monoxide
possible only in an industrial environment. Carbon dioxide and hydrogen are formed, the principle of their separation is described above.

THE INVENTION HAS THE FOLLOWING ADVANTAGES
The heat obtained from the oxidation of gases can be used directly on site, and hydrogen and oxygen are obtained from the disposal of waste steam and process water.
Low water consumption when generating electricity and heat.
The simplicity of the way.
Significant energy savings as it is spent only on warming up the starter to the established thermal regime.
High productivity of the process, because dissociation of water molecules lasts tenths of a second.
Explosion and fire safety of the method, because in its implementation, there is no need for containers for collecting hydrogen and oxygen.
During the operation of the installation, water is purified many times, being converted into distilled water. This eliminates sediments and limescale, which increases the service life of the installation.
The installation is made of ordinary steel; with the exception of boilers made of heat-resistant steels with lining and shielding of their walls. That is, no special expensive materials are required.
The invention can find application in
industry by replacing hydrocarbon and nuclear fuel in power plants with cheap, widespread and environmentally friendly water, while maintaining the power of these plants.
Combustion of hydrogen
Hydrogen, therefore, gives birth to water. Water is obtained by burning hydrogen - by combining hydrogen with oxygen. A very large amount of energy is released during the reaction.
2H2 + O2 = 2H2O + Q
This means that hydrogen can be used as fuel. And as with any fuel, hydrogen must be handled with care.
We get hydrogen by the reaction of zinc with hydrochloric acid.
We ignite hydrogen at the end of the gas outlet tube. At first, the flame is barely noticeable (hydrogen does not color the flame). Gradually the glass tube becomes hot and the flame turns yellow: the sodium compounds that make up the glass color the flame.
Fig. 2. Combustion of hydrogen
So hydrogen is fuel. Jet engines can run on hydrogen and oxygen. The heat of reaction of hydrogen combustion is used for welding and cutting metals. When hydrogen burns in pure oxygen, the temperature reaches 2800 ° C. This flame melts quartz and most metals. It is important that hydrogen is an environmentally friendly fuel. the product of its combustion is water.
CLAIM
Method for producing hydrogen and oxygen from water vapor
, including passing this steam through an electric field, characterized in that they use superheated water steam with a temperature
500 - 550 o C
, passed through a high-voltage direct current electric field to dissociate vapor and separate it into hydrogen and oxygen atoms.
I have long wanted to do a similar thing. But further experiments with a battery and a pair of electrodes did not reach. I wanted to make a full-fledged apparatus for the production of hydrogen, in quantities in order to inflate a balloon. Before making a full-fledged apparatus for electrolysis of water at home, I decided to check everything on the model.
The general scheme of the electrolyzer looks like this.
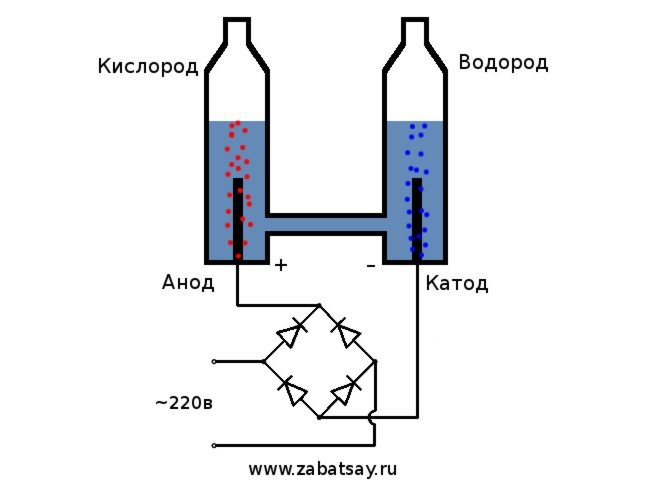

This model is not suitable for full daily use. But we managed to test the idea.
So I decided to use graphite for the electrodes. An excellent source of graphite for electrodes is the trolleybus collector. There are plenty of them at the end stops. It must be remembered that one of the electrodes will collapse.


We saw and finalize with a file. The intensity of electrolysis depends on the strength of the current and the area of the electrodes.


Wires are attached to the electrodes. The wires must be carefully insulated.


For the case of the electrolytic cell model, plastic bottles are quite suitable. Holes are made in the cover for pipes and wires.


Everything is thoroughly coated with sealant.


Cut-off bottle necks are suitable for connecting two containers.


They need to be joined together and the seam must be melted.


The nuts are made from bottle caps.


Holes are made in two bottles at the bottom. Everything is connected and carefully filled with sealant.


We will use a 220V household network as a voltage source. I want to warn you that this is a rather dangerous toy. So, if you do not have sufficient skills or there are doubts, then it is better not to repeat. In the household network, we have an alternating current, for electrolysis it must be straightened. A diode bridge is perfect for this. The one in the photo was not powerful enough and quickly burned out. The best option was the Chinese MB156 diode bridge in an aluminum case.


The diode bridge gets very hot. Active cooling will be required. A cooler for a computer processor is perfect. A junction box of a suitable size can be used for the enclosure. Sold in electrical goods.


Several layers of cardboard must be placed under the diode bridge.


The necessary holes are made in the cover of the junction box.


This is what the assembled unit looks like. The electrolyzer is powered from the mains, the fan is powered by a universal power source. A baking soda solution is used as an electrolyte. Here it must be remembered that the higher the concentration of the solution, the higher the reaction rate. But at the same time, the heating is also higher. Moreover, the reaction of sodium decomposition at the cathode will contribute to the heating. This reaction is exothermic. As a result, hydrogen and sodium hydroxide will be formed.


The device in the photo above was very hot. It had to be turned off periodically and wait until it cools down. The heating problem was partially solved by cooling the electrolyte. For this I used a tabletop fountain pump. A long tube runs from one bottle to another through a pump and a bucket of cold water.


The relevance of this issue today is quite high due to the fact that the sphere of using hydrogen is extremely extensive, and in its pure form it is practically not found anywhere in nature. That is why several techniques have been developed that allow the extraction of this gas from other compounds through chemical and physical reactions. This is discussed in the article above.
Lesson Practical work "Obtaining hydrogen and studying its properties."
Lesson 31 Grade 8 -
Subject:
Practical work No. 4 Obtaining hydrogen and studying its properties.
Date ____________20
MBOU "S (K) OSH №16", chemistry teacher Berezinskaya A.A.
Purpose:
- improve experimental skills - techniques for working with laboratory equipment and substances; the ability to observe, draw conclusions, draw up the results of practical work in notebooks;
- work on the development of skills in the skillful handling of fire, hazardous substances.
- the ability to draw up equations of chemical reactions, the ability to draw conclusions, follow safety rules;
- broadening the horizons of students, building respect for the history of science.
- development of ideas about a healthy lifestyle in blocks: "Chemistry in everyday life - safe behavior."
Corrective goals:
correction and development of coherent oral and written speech, correction and development of motor memory, development of the ability to draw conclusions.
Equipment:
- laboratory rack with foot, test tube holder, test tube rack, dosing spoon, filter paper
- spirit lamp, matches
- automatic Kiryushkin device for obtaining gases, 3 test tubes, crystallizer with water
Reagents:
zinc granules, hydrochloric acid (diluted), copper (II) oxide.
Lesson type
: practical lesson (virtual laboratory)
Safety regulations:
Working with a spirit lamp; work with glass; Checking the device for leaks.
Progress:
I. Preparation for practical work.
- Safety briefing when working with dry fuel.
- Technical briefing on how to carry out practical work.
II. Knowledge update
- What starting materials will we use to obtain hydrogen?
- Does the reaction mixture need to be heated?
- What to look for when recording observations?
- What device will we use to produce hydrogen?
- What methods can be used to collect hydrogen, why?
Acquaintance with the instruction: tutorial page ________
III. Practical work (watching the video: Hydrogen production.)
III. Consolidation of knowledge, abilities, skills.
After carrying out the work, draw a conclusion, write down all the results in a notebook.
Homework: § ________.
Practical work No. 4. Production of hydrogen and study of its properties.
I am familiar with the safety rules
Purpose:
learn to receive, collect hydrogen; study the physical and chemical properties of hydrogen.
Equipment:
laboratory rack with a foot, a holder for test tubes, a rack for test tubes, a dosing spoon, filter paper, an alcohol lamp, matches, an automatic Kiryushkin device for obtaining gases, 3 test tubes, a crystallizer with water.
Reagents:
zinc granules, hydrochloric acid (diluted), copper (II) oxide.
Progress
1. A method of producing hydrogen - the interaction of active metals with acids.
Zn + 2HCl = ZnCl2 + H2 ↑ + Q - under normal conditions
Observations:
- the reaction of the interaction of zinc granules with hydrochloric acid proceeds slowly at first, then very violently, the test tube heats up
- colorless gas escapes from the gas outlet pipe
- when the resulting solution is evaporated, a white powder remains on the glass plate
2. Devices for obtaining and collecting hydrogen
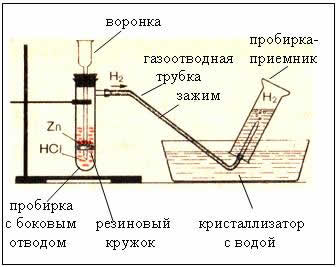

Fig. The device for producing hydrogen is automatic, which allows you to stop the reaction at any time using a clamp (Kiryushkin's device).
Collecting gas by water displacement is possible because hydrogen is slightly soluble in it.
- therefore, hydrogen is lighter than air
3. Detection of hydrogen - checking it for purity
Observations:
- when the first portion of gas is burned, a sharp barking sound is heard
- when burning the second portion of gas, a light cotton is heard Figure 5
"P-groin"
4. The property of hydrogen is an active reducing agent
Observations:
- the powder changes color from black to copper
- colorless liquid droplets appear on the walls of the test tube
Output:
One of the ways to obtain hydrogen in the laboratory is the interaction of zinc with dilute hydrochloric acid, which forms a salt (zinc chloride) and hydrogen. Hydrogen is a colorless gas, odorless, slightly soluble in water, lighter than air, explosive when mixed with air, reduces metals from their oxides.
3
Household hydrogen production
Electrolyzer selection
To obtain an element of the house, you need a special apparatus - an electrolyser. There are many options for such equipment on the market; the devices are offered by both well-known technology corporations and small manufacturers. Branded units are more expensive, but the build quality is higher.
The home appliance is small and easy to use. Its main details are:
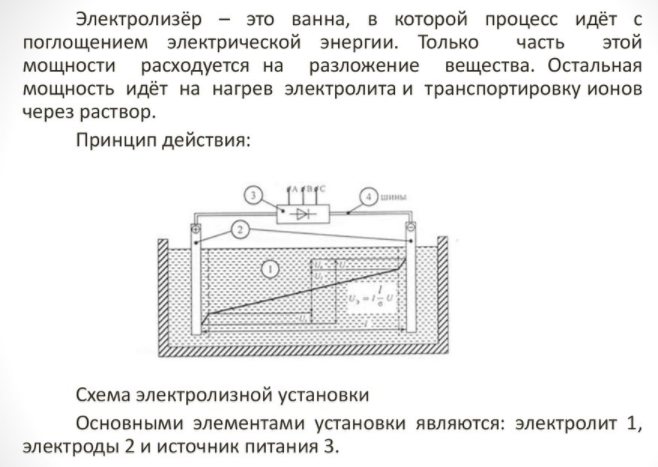

Electrolyzer - what is it
- reformer;
- cleaning system;
- fuel cells;
- compressor equipment;
- a container for storing hydrogen.
Simple tap water is taken as raw material, and electricity comes from a regular outlet. Solar-powered units save on electricity.
Home hydrogen is used in heating or cooking systems. And also they enrich the fuel-air mixture in order to increase the power of the car's engines.
Making an apparatus with your own hands
It is even cheaper to make the device yourself at home. A dry cell looks like a sealed container, which consists of two electrode plates in a container with an electrolytic solution. The World Wide Web offers a variety of assembly schemes for devices of different models:
- with two filters;
- with top or bottom arrangement of the container;
- with two or three valves;
- with galvanized board;
- on the electrodes.
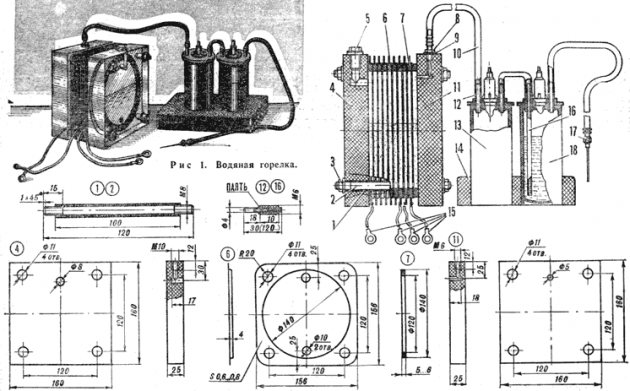

Electrolysis device diagram
It is not difficult to create a simple device for producing hydrogen. It will require:
- sheet stainless steel;
- transparent tube;
- fittings;
- plastic container (1.5 l);
- water filter and non-return valve.
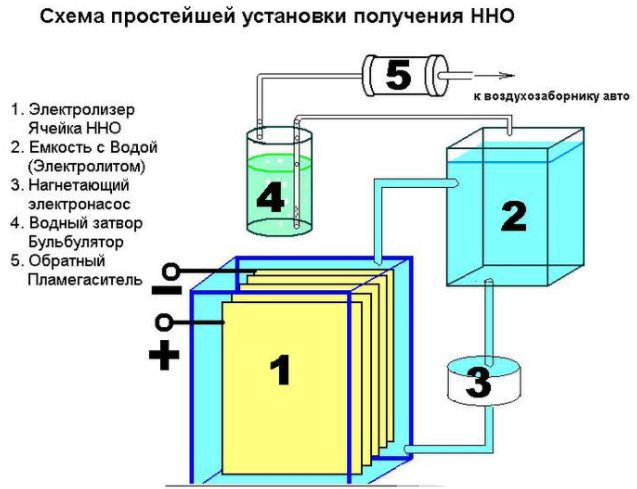

The device of a simple device for producing hydrogen
In addition, various hardware will be needed: nuts, washers, bolts. The first step is to cut the sheet into 16 square compartments, cut off a corner from each of them. In the opposite corner from it, it is required to drill a hole for bolting the plates. To ensure constant current, the plates must be connected according to the plus – minus – plus – minus scheme. These parts are isolated from each other with a tube, and at the connection with a bolt and washers (three pieces between the plates). On the plus and minus, 8 plates are planted.
When properly assembled, the ribs of the plates will not touch the electrodes. The assembled parts are lowered into a plastic container. At the point where the walls touch, two mounting holes are made with bolts. Install a safety valve to remove excess gas. Fittings are mounted in the container lid and the seams are sealed with silicone.
Testing the apparatus
To test the device, perform several actions:

Hydrogen production scheme
- Fill with liquid.
- Covering with a lid, connect one end of the tube to the fitting.
- The second is immersed in water.
- Connect to a power source.
After plugging the device into an outlet, after a few seconds, the electrolysis process and precipitation will be noticeable.
Pure water does not have good electrical conductivity. To improve this indicator, you need to create an electrolytic solution by adding an alkali - sodium hydroxide. It is found in pipe cleaning compounds like the Mole.
How the device works
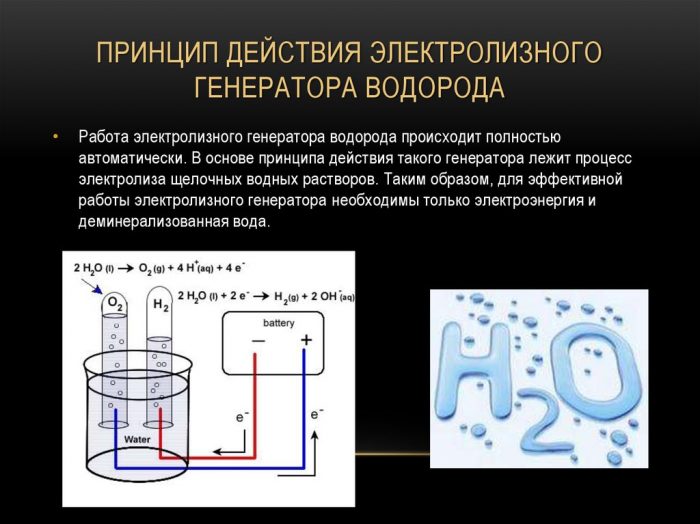
The electrolyzer consists of several metal plates immersed in a sealed container with distilled water.
The body itself has terminals to connect the power source and there is a bushing through which gas is discharged.
The operation of the device can be described as follows: an electric current is passed through distilled water between plates with different fields (one has an anode, the other has a cathode), splits it into oxygen and hydrogen.
Depending on the area of the plates, the electric current has its own strength, if the area is large, then a lot of current passes through the water and more gas is released. The connection diagram of the plates is alternate, first plus, then minus, and so on.
The electrodes are recommended to be made of stainless steel, which does not react with water during the electrolysis process. The main thing is to find high quality stainless steel. Better to make the distance between the electrodes small, but so that the gas bubbles can easily move between them. It is better to make fasteners from the corresponding metal as the electrodes.
In this embodiment, the device includes 16 plates, they are located within 1 mm from each other.
Due to the fact that the plates have a fairly large surface area and thickness, it will be possible to pass high currents through such a device, but the metal will not heat up. If you measure the capacitance of the electrodes in air, then it will be 1nF, this set uses up to 25A in plain water from the mains.
To collect a hydrogen generator with your own hands, you can use a food container, since its plastic is heat-resistant. Then you need to lower the electrodes for collecting gas with hermetically insulated connectors, a cover and other connections into the container.
If you use a container made of metal, then in order to avoid a short circuit, the electrodes are attached to plastic. On both sides of the copper and brass fittings, two connectors are installed (fitting - mount, assemble) for gas extraction. Contact connectors and fittings must be firmly fixed using silicone sealant.
You can also make a gas generator at home. The technique is detailed here:
Methods for producing hydrogen
Hydrogen is a colorless and odorless gaseous element with a density of 1/14 relative to air. In a free state, it is rare. Usually hydrogen is combined with other chemical elements: oxygen, carbon.
Hydrogen production for industrial needs and power engineering is carried out by several methods. The most popular are:
- electrolysis of water;
- concentration method;
- low temperature condensation;
- adsorption.
Hydrogen can be isolated not only from gaseous or water compounds. Hydrogen is produced by exposing wood and coal to high temperatures, as well as by processing biowaste.
Atomic hydrogen for power engineering is obtained using the method of thermal dissociation of a molecular substance on a wire made of platinum, tungsten or palladium. It is heated in a hydrogen atmosphere under a pressure of less than 1.33 Pa. And also radioactive elements are used to obtain hydrogen.

Thermal dissociation
Electrolysis method
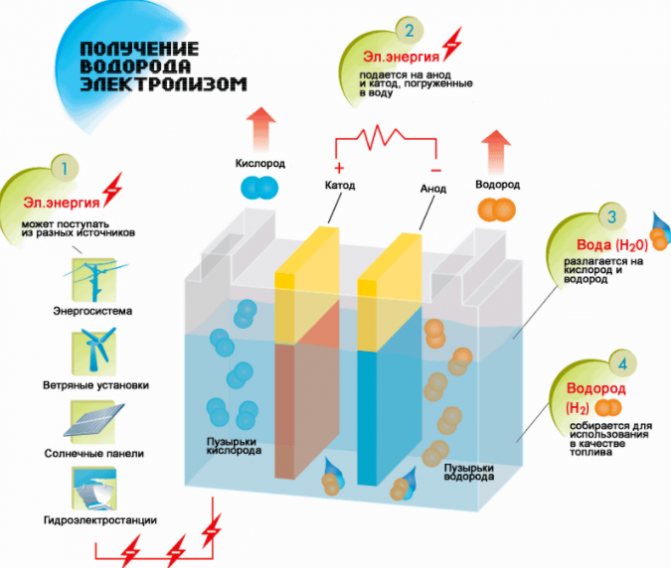
The simplest and most popular method of hydrogen evolution is water electrolysis. It allows the production of practically pure hydrogen.Other advantages of this method are:

The principle of operation of the electrolysis hydrogen generator
- availability of raw materials;
- receiving an element under pressure;
- the ability to automate the process due to the lack of moving parts.
The procedure for splitting a liquid by electrolysis is the reverse of the combustion of hydrogen. Its essence is that under the influence of direct current, oxygen and hydrogen are released on the electrodes dipped in an aqueous electrolyte solution.
An additional advantage is considered to be the production of by-products with industrial value. Thus, a large amount of oxygen is needed to catalyze technological processes in the energy sector, clean up soil and water bodies, and dispose of household waste. Heavy water obtained during electrolysis is used in power engineering in nuclear reactors.
Hydrogen production by concentration
This method is based on the separation of an element from gas mixtures containing it. So, the largest part of the substance produced in industrial volumes is extracted using steam reforming of methane. Hydrogen extracted in this process is used in energy, oil refining, rocket-building industries, as well as for the production of nitrogen fertilizers. The process of obtaining H2 is carried out in different ways:
- short-cycle;
- cryogenic;
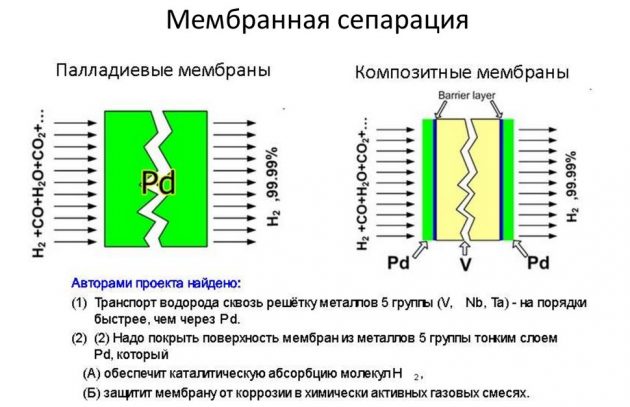
- membrane.
The latter method is considered the most effective and less costly.

Low temperature condensation
This method of obtaining H2 consists in strong cooling of gaseous compounds under pressure. As a result, they are transformed into a two-phase system, which is subsequently separated by a separator into a liquid component and a gas. Liquid media are used for cooling:
- water;
- liquefied ethane or propane;
- liquid ammonia.


This procedure is not as easy as it sounds. It will not be possible to cleanly separate hydrocarbon gases at once. Some of the components will leave with gas taken from the separation compartment, which is not economical. The problem can be solved by deep cooling of the raw material before separation. But this requires a lot of energy.
In modern low temperature condenser systems, demethanization or deethanization columns are additionally provided. The gas phase is removed from the last separation stage, and the liquid is sent to the distillation column with a stream of raw gas after heat exchange.
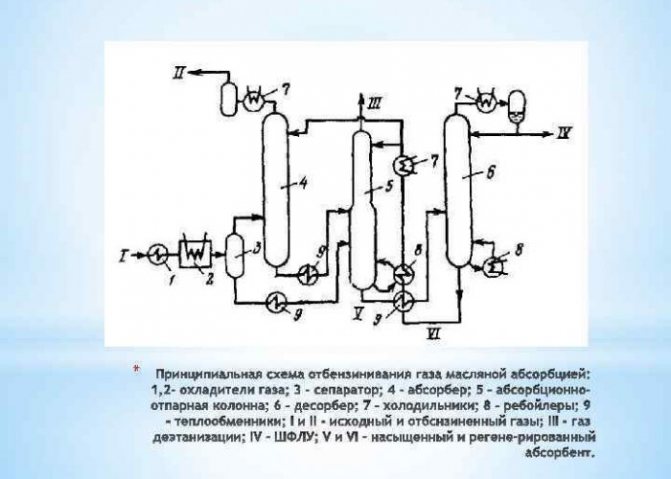
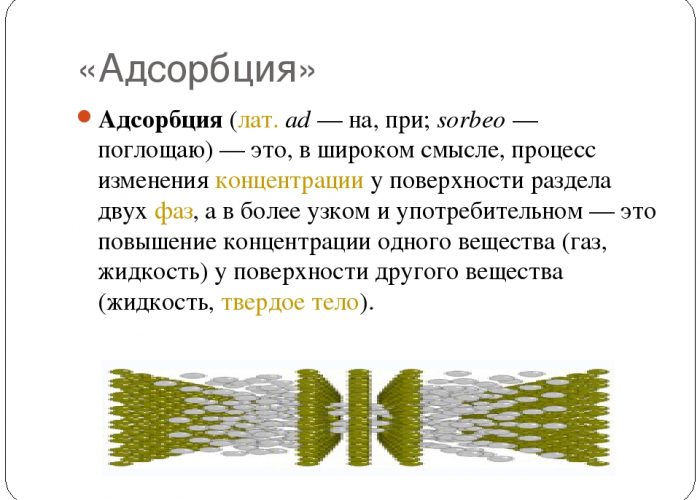
Adsorption method
During adsorption, to release hydrogen, adsorbents are used - solids that absorb the necessary components of the gas mixture. Activated carbon, silicate gel, zeolites are used as adsorbents. To carry out this process, special devices are used - cyclic adsorbers or molecular sieves. When implemented under pressure, this method can recover 85 percent hydrogen.
If we compare adsorption with low-temperature condensation, we can note a lower material and operating cost of the process - on average, by 30 percent. Hydrogen is produced by adsorption for power engineering and with the use of solvents. This method allows the extraction of 90 percent of H2 from the gas mixture and obtaining the final product with a hydrogen concentration of up to 99.9%.